Optional
Problems to turn in:
Problem 10.1 Fermi, pg 10, number 1
Problem 10.2 Fermi, pg 10, number 4
Problem 10.3 Fermi, pg 45, number 3
Problem 10.4 A vertical cylinder 2.5 m tall is filled with 0.08 moles of an ideal gas at STP. Next, a piston of mass 1.5 kg is added to one end of the cylinder compressing the gas somewhat. It seals the cylinder but is free to move vertically. (1)What is the equilibrium height of the piston assuming that the gas is isothermal. (2)The piston is now displaced slightly and released. What is the frequency of oscillation?
Problem 10.5 Knight 19-72
Problem 10.6 Entropy is a state variable.
- a) The left-hand p-V diagram shows two
ways to bring an ideal gas from point a
to point c: directly along the isotherm ac,
or constant volume to point
b, then constant pressure to point c.
By directly calculating Delta S for the two paths,
show that the change in
entropy is the same for both.
- b) Optional: not for credit, but try it if you like. You could do the same for the right-hand diagram, where the isotherm is replaced by an adiabat (and this changes the relations among the various p's and V's). Instead,
use the fact that S is a state variable to derive the form of the adiabat, that is, the relation between p and V. Note: we aren't pretending to discover this -- it helps in your manipulations to know what you are after. - b) Optional: not for credit, but try it if you like. You could do the same for the right-hand diagram, where the isotherm is replaced by an adiabat (and this changes the relations among the various p's and V's). Instead,
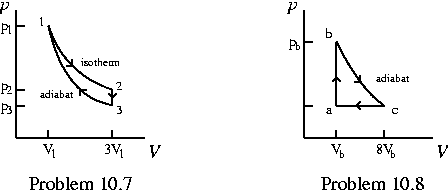
Problem 10.7 An ideal diatomic gas is taken through the cycle shown in the figure below. Determine for all three processes, in terms of p1, V1, T1, and R,
- a) p2, p3, T3
- b) W, Q, Delta U, and Delta S, all per mole.
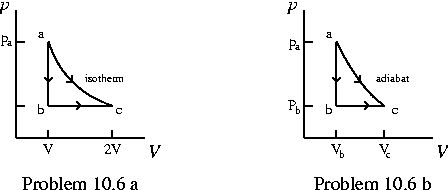
Problem 10.8 One mole of a monatomic ideal gas is taken through the reversible cycle shown in the (not-to-scale) figure below. Process bc is an adiabatic expansion, with pb=10.0 atm and Vb=1.00×10 -3 m3. Find:
- a) the heat added to the gas,
- b) the heat leaving the gas,
- c) the net work done by the gas, and
- d) the efficiency of the cycle.
- e) What would the maximum possible efficiency be for
any engine operating between the same maximum and minimum temperatures as this one? - b) the heat leaving the gas,